A 6 m3 tank contains helium at 400 K and is evacuated from atmospheric pressure to a pressure of 740 mm Hg vacuum. Determine the mass of the helium remaining in the tank?

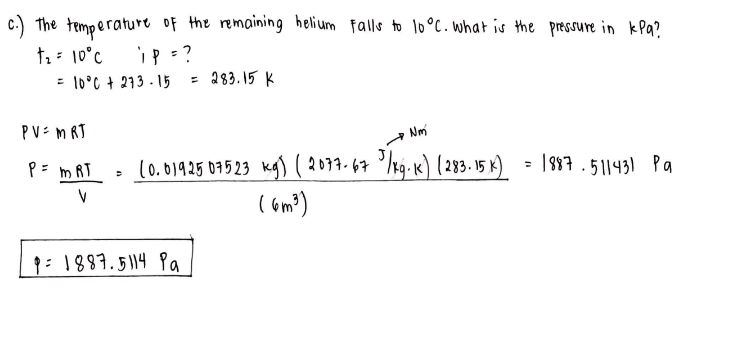
A 6 m3 tank contains helium at 400 K and is evacuated from atmospheric pressure to a pressure of 740 mm Hg vacuum. Determine the mass of the helium remaining in the tank?

